Does Ozempic Reduce Heart Attacks?
Jan 22, 2026
Semaglutide and Heart Risk: What the Research Shows
Can Ozempic (semaglutide) reduce cardiovascular risk? Is it due to the weight loss? Is it due to the medication alone? What if you are not diabetic? What happens if you are not diabetic and don't lose weight? Will you still reduce heart disease risk?
As it turns out... Yes! To all of these questions! Let's dive deep into this topic. First, the bullet points and highlights, then we will dive deep into the trials! Here's the quick infographic.
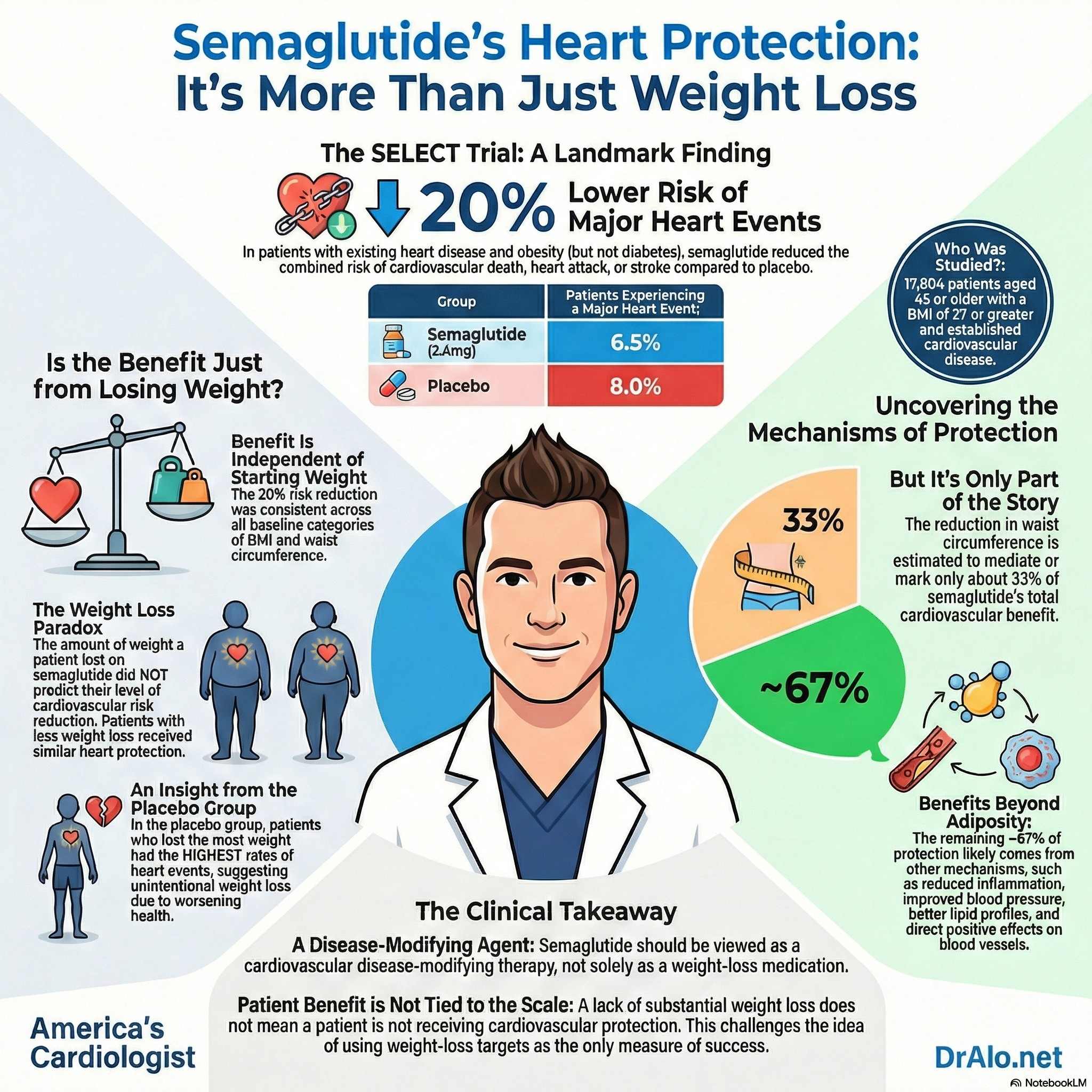
Biggest Study: SELECT (Semaglutide in People Without Diabetes)
The most important evidence comes from a major clinical trial called SELECT.
Who was in the SELECT study?
Researchers enrolled 17,604 adults who:
- were 45 years or older
- had overweight or obesity (BMI ≥ 27)
- already had heart disease (such as a prior heart attack, stroke, or peripheral artery disease)
- did not have diabetes
Participants were randomly assigned to receive either:
- semaglutide 2.4 mg weekly, or
- a placebo (an injection with no active medication)
They were followed for about 40 months (over 3 years).
What did SELECT find?
People who took semaglutide had a:
✅ 20% lower risk of major cardiovascular events — meaning fewer:
- cardiovascular deaths
- heart attacks
- strokes
This was a landmark result because it showed semaglutide can reduce heart risk even when diabetes is not part of the picture.
Why this result is especially impressive
Most people in the SELECT trial were already receiving strong heart-protection medications:
- ~90% were taking statins
- many were on blood pressure medicines
- many were on antiplatelet therapy (like aspirin)
So semaglutide helped on top of already excellent medical care.
Benefits Go Beyond Weight Loss
A key discovery from later analyses is that semaglutide’s heart benefits are not only about losing weight.
In a detailed follow-up analysis of SELECT, researchers found:
✅ People got similar cardiovascular protection whether they:
- lost ≥5% of body weight, or
- lost very little weight (or even none)
That’s a major finding because it suggests semaglutide may protect the heart through multiple pathways, such as:
- lowering inflammation
- improving blood vessel function
- reducing blood pressure
- improving cholesterol and lipid markers
In SELECT, semaglutide also improved several risk indicators, including lower blood pressure and a major drop in C-reactive protein (CRP) — a marker linked with inflammation and heart risk.
What About People With Diabetes?
Semaglutide has also shown strong cardiovascular benefits in people with type 2 diabetes, where heart risk is especially high.
One of the key trials, SUSTAIN 6, found that semaglutide lowered the risk of major cardiovascular events in high-risk patients with diabetes.
These results helped establish semaglutide as not just a diabetes or weight-loss medication, but also a cardiovascular risk-reducing treatment in the right populations.
Oral Semaglutide Also Helps: The SOUL Trial
Some people prefer pills instead of injections. The SOUL trial tested oral semaglutide in people with type 2 diabetes who were at high cardiovascular risk.
The key result:
✅ Oral semaglutide reduced major cardiovascular events by 14% compared with placebo.
This strengthened the evidence that semaglutide’s cardiovascular protection applies across different formulations — not only injections.
Heart Failure: Even Stronger Protection in Some Patients
Semaglutide may be especially helpful for people who have both:
- obesity/overweight, and
- heart failure
A prespecified analysis of SELECT looked at participants who had heart failure at the start of the trial and found:
✅ semaglutide reduced major cardiovascular events by about 28% in this subgroup and also reduced serious heart failure-related outcomes (like hospitalizations).
These benefits were seen in different types of heart failure, including both:
- reduced ejection fraction (weaker pumping)
- preserved ejection fraction (stiffer filling)
What the FDA Approvals Reflect
As evidence has grown, FDA-approved semaglutide products now include cardiovascular indications depending on the patient population:
- Wegovy (2.4 mg injection): approved to reduce cardiovascular risk in people with established heart disease and overweight/obesity.
- Ozempic (injection): approved for cardiovascular risk reduction in adults with type 2 diabetes and established cardiovascular disease (and also has kidney-related risk reduction indications in certain patients).
- Rybelsus (oral tablet): approved for type 2 diabetes, and cardiovascular outcome evidence continues to expand with trials like SOUL.
Safety and Side Effects (What People Should Know)
The most common reason people stop semaglutide is stomach-related side effects, such as:
- nausea
- vomiting
- diarrhea
- constipation
In SELECT, more people stopped semaglutide than placebo due to these effects. It's important to note that these are rare and usually improve if you are able to stay on the medication. Rarely, are these bad enough to require someone to stop the medications.
However, overall serious complications were not more common in semaglutide users — and in some studies, serious adverse events occurred slightly less often in the semaglutide group.
The big takeaway is that semaglutide’s heart benefits are real — but the decision to use it should involve a discussion of tolerability, costs, and individual health risks.
Does Ozempic reduce heart attack risk?
Semaglutide is now backed by strong evidence showing it can do more than help with weight or diabetes:
✅ It can lower the risk of heart attacks, strokes, and cardiovascular death, including in people without diabetes who already have heart disease.
Even more surprising:
✅ Much of the cardiovascular protection appears to happen even when weight loss is small, suggesting semaglutide works through additional “heart-protective” mechanisms like lowering inflammation and improving blood vessel health.
This represents a major shift in how doctors may approach cardiovascular prevention in people with overweight/obesity and existing heart disease.
Benefits Beyond Diabetes
Semaglutide is a medication that was first developed to treat type 2 diabetes, and later became widely used for weight loss. But in recent years, research has shown something even bigger:
✅ Semaglutide can lower the risk of serious heart problems, including heart attacks and strokes — even in people without diabetes.
This matters because heart disease remains the leading cause of death worldwide, and excess weight strongly increases cardiovascular risk. Until recently, weight-loss medications had not clearly been shown to directly prevent heart attacks or strokes. Semaglutide appears to be different.
Details For Those Who Want To Dive Deeper:
Semaglutide and Cardiovascular Risk: What the Evidence Shows
Semaglutide, a medication originally developed for weight management and diabetes treatment, has emerged as a powerful tool for reducing cardiovascular risk. The landmark SELECT trial demonstrated that weekly semaglutide 2.4 mg reduces major cardiovascular events by 20% in people with obesity or overweight and established heart disease—even in those without diabetes.[1] This finding represents a paradigm shift in cardiovascular prevention, as it's the first time a weight-loss medication has been proven to directly reduce heart attacks, strokes, and cardiovascular deaths.
The implications extend far beyond weight management. With over half the global population projected to have overweight or obesity by 2035, and excess weight contributing to millions of cardiovascular deaths annually, semaglutide offers a new approach to addressing one of medicine's most pressing challenges. Unlike previous weight-loss interventions that failed to demonstrate cardiovascular benefits, semaglutide's effects appear early and persist over time, suggesting mechanisms beyond simple weight reduction.
The SELECT Trial: Design and Key Findings
The SELECT trial enrolled 17,604 patients aged 45 or older with a BMI of 27 or greater and established cardiovascular disease (prior heart attack, stroke, or symptomatic peripheral artery disease), but critically, none had diabetes.[1] This design was intentional—researchers wanted to understand whether semaglutide's cardiovascular benefits extended beyond its known effects in diabetic populations. Patients were randomized to receive either weekly subcutaneous semaglutide 2.4 mg or placebo, with the dose gradually increased over 16 weeks to minimize gastrointestinal side effects.
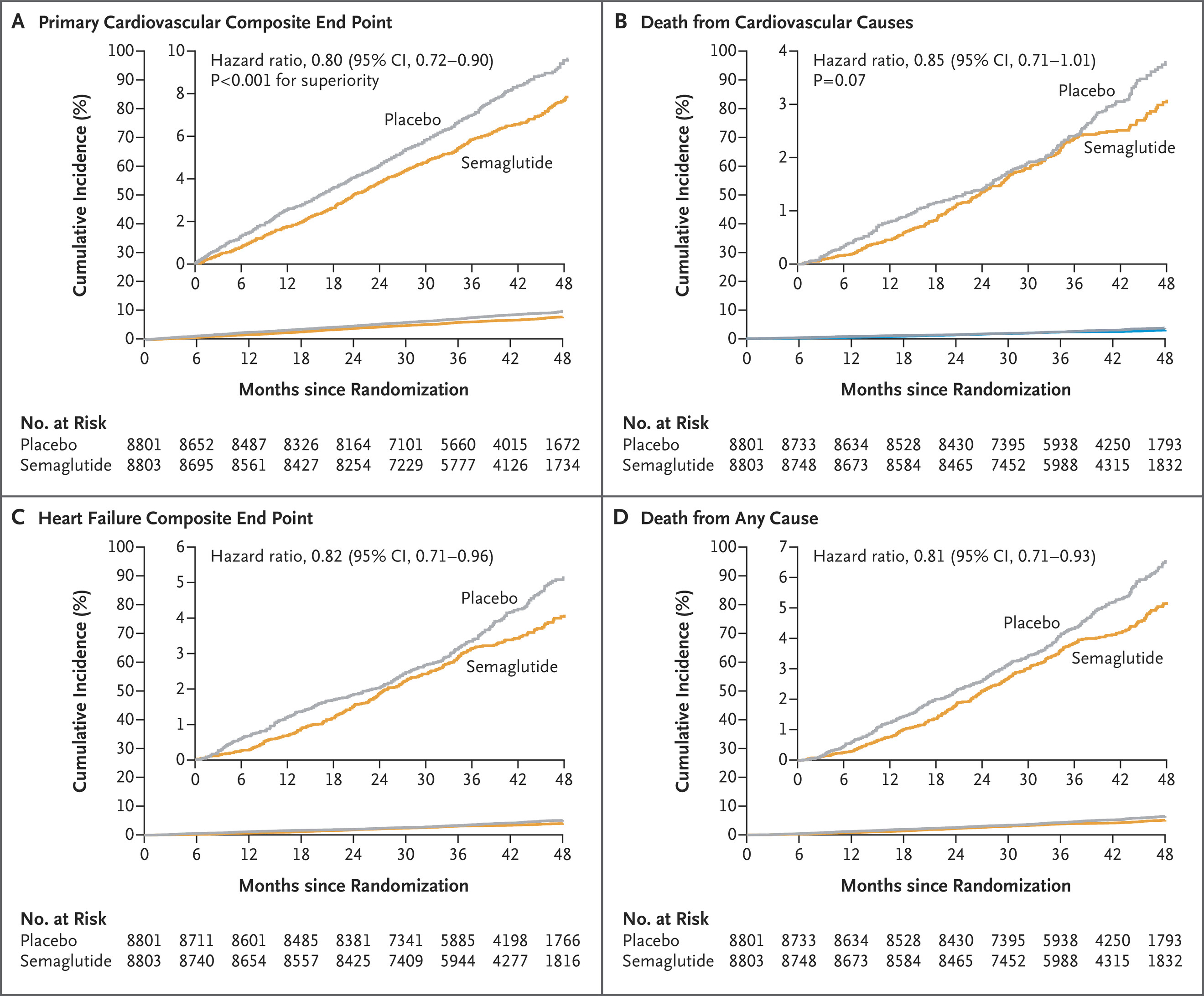
Figure 1. Time-to-First-Event Analysis for Primary and Confirmatory Secondary Efficacy End Points.
The figure above illustrates the trial's primary findings across four key cardiovascular outcomes. The top panel shows the 20% reduction in the composite primary endpoint (cardiovascular death, nonfatal heart attack, or nonfatal stroke), with curves separating early and maintaining divergence throughout the nearly 40-month follow-up period. While cardiovascular death alone didn't reach statistical significance (middle-left panel), consistent directional benefits appeared across heart failure outcomes and all-cause mortality, suggesting broad cardiovascular protection.
What makes these results particularly compelling is the patient population's baseline characteristics. Most participants were already receiving excellent cardiovascular care—90% were on statins, 86% on antiplatelet agents, and the majority on blood pressure medications. Despite this optimized medical therapy, semaglutide provided additional benefit. The medication also produced a mean 9.4% weight loss and improvements in blood pressure (−3.3 mm Hg systolic), inflammatory markers (−37.8% high-sensitivity CRP), and lipid profiles—all achieved on top of existing treatments. Gastrointestinal side effects led to treatment discontinuation in 10% of semaglutide patients versus 2% on placebo, though serious adverse events were actually less frequent with semaglutide (33.4% vs 36.4%).
Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes.
Beyond SELECT's findings in people without diabetes, semaglutide has demonstrated cardiovascular benefits across diverse patient populations, including those with type 2 diabetes and varying degrees of heart failure. The SUSTAIN 6 trial showed that subcutaneous semaglutide reduced major adverse cardiovascular events by 26% in patients with type 2 diabetes and established cardiovascular disease (hazard ratio 0.74, 95% CI 0.58-0.95). This reduction was driven primarily by fewer nonfatal strokes (39% reduction) and nonfatal heart attacks (26% reduction), establishing semaglutide's role in cardiovascular protection for diabetic patients.
Oral Semaglutide Extends Cardiovascular Benefits
The recently completed SOUL trial demonstrated that oral semaglutide—a more convenient formulation—also reduces cardiovascular risk in high-risk patients with type 2 diabetes. The trial enrolled 9,650 participants with diabetes and either atherosclerotic cardiovascular disease, chronic kidney disease, or both, following them for an average of 47.5 months. Oral semaglutide reduced major adverse cardiovascular events by 14% compared to placebo (hazard ratio 0.86, 95% CI 0.77-0.96). Interestingly, nonfatal heart attack showed the largest risk reduction among the three primary outcome components, contrasting with the earlier PIONEER 6 trial where cardiovascular death was the dominant benefit—likely because SOUL's longer follow-up and larger sample size (approximately three times that of PIONEER 6) allowed more comprehensive assessment of cardiovascular outcomes.
Heart Failure Populations Show Consistent Benefits
For patients with both obesity and heart failure, semaglutide's cardiovascular protection appears particularly robust. A prespecified analysis of SELECT examined 4,286 participants (24.3% of the total trial population) who had investigator-defined heart failure at enrollment. Among these patients, semaglutide reduced major adverse cardiovascular events by 28% (hazard ratio 0.72, 95% CI 0.60-0.87)—an even greater benefit than seen in the overall trial population. The composite heart failure endpoint (cardiovascular death or hospitalization/urgent visit for heart failure) showed a 21% reduction (hazard ratio 0.79).
These benefits extended across heart failure subtypes. Patients with heart failure with reduced ejection fraction experienced a 35% reduction in major cardiovascular events (hazard ratio 0.65, 95% CI 0.49-0.87), while those with heart failure with preserved ejection fraction saw a 31% reduction (hazard ratio 0.69, 95% CI 0.51-0.91). Notably, patients with reduced ejection fraction had higher absolute event rates, meaning the medication prevented more events in this higher-risk group. The benefits appeared consistent regardless of baseline age, sex, BMI, New York Heart Association functional class, or diuretic use.
Cardiovascular Protection Independent of Glucose Control
One of the most intriguing findings from the SELECT trial involves semaglutide's mechanism of cardiovascular benefit. Among participants without diabetes, cardiovascular risk reduction was consistent across all baseline HbA1c subgroups (less than 5.7%, 5.7% to 6.0%, and 6.0% to 6.5%) and across categories of HbA1c change from baseline to 20 weeks. This suggests that semaglutide confers cardiovascular protection through mechanisms beyond glucose lowering—likely related to weight reduction, blood pressure improvement, and anti-inflammatory effects. The 57.3% reduction in high-sensitivity C-reactive protein observed with oral semaglutide 50 mg would move patients from high cardiovascular risk (greater than 3 mg/dL) to intermediate risk (1-3 mg/dL), independent of glycemic changes.
Meta-Analyses Confirm Broad Cardiovascular Benefits
Multiple systematic reviews have synthesized semaglutide's cardiovascular effects across trials. A 2025 meta-analysis of four randomized controlled trials involving 27,617 participants demonstrated a 19% reduction in major adverse cardiovascular events (relative risk 0.81, 95% CI 0.74-0.88), with additional benefits for cardiovascular death and nonfatal heart attack. Another comprehensive meta-analysis revealed significant reductions in hospitalization for heart failure (76% reduction, relative risk 0.24), death from cardiovascular causes (17% reduction), coronary revascularization (24% reduction), and all-cause mortality (21% reduction). Interestingly, stroke reduction reached statistical significance only in patients with diabetes (35% reduction, relative risk 0.65), suggesting potential differences in mechanism or baseline risk profiles between diabetic and non-diabetic populations.
FDA Approvals Reflect Evidence Base
Based on these cardiovascular outcome trials, the FDA has approved three semaglutide formulations for cardiovascular risk reduction. Ozempic (subcutaneous semaglutide) is approved for reducing major adverse cardiovascular events in adults with type 2 diabetes and established cardiovascular disease, as well as reducing the risk of sustained kidney function decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease. Rybelsus (oral semaglutide) is approved for reducing major adverse cardiovascular events in adults with type 2 diabetes. Wegovy (subcutaneous semaglutide 2.4 mg) is approved for reducing major adverse cardiovascular events in adults with established cardiovascular disease and obesity or overweight—the first weight-management medication to receive this indication.
Safety Considerations
While semaglutide demonstrates clear cardiovascular benefits, treatment discontinuation rates merit attention. Meta-analyses show that semaglutide increases the likelihood of discontinuing treatment (relative risk 1.67, 95% CI 1.27-2.21), primarily due to gastrointestinal adverse events. However, this must be weighed against the medication's ability to reduce serious adverse events overall (relative risk 0.91, 95% CI 0.88-0.94). The trade-off between tolerability and cardiovascular protection represents an important consideration for patient counseling and shared decision-making.
Semaglutide reduces cardiovascular risk independent of weight loss, with similar cardiovascular benefits observed in patients who did not lose weight compared to those who did. A prespecified analysis of the SELECT trial examined the relationship between changes in adiposity measures (body weight and waist circumference) and cardiovascular outcomes among 17,604 patients with obesity or overweight and established cardiovascular disease but without diabetes.[1] The analysis revealed that cardiovascular outcomes were similar among semaglutide-treated patients who did or did not lose at least 5% of their baseline body weight, and that semaglutide consistently reduced major adverse cardiovascular events across the full spectrum of baseline body mass index values.
The findings challenge the assumption that weight loss is the primary mechanism by which semaglutide confers cardiovascular protection. While waist circumference reduction showed a modest association with cardiovascular benefit, it accounted for only 33% of semaglutide's treatment effect on major adverse cardiovascular events.[1] This suggests that the majority of cardiovascular benefit occurs through mechanisms beyond adiposity reduction—including direct effects on inflammation, endothelial function, blood pressure, and lipid metabolism.
SELECT Landmark Study in Detail
The SELECT trial enrolled patients with a mean BMI of 33.3 and preexisting cardiovascular disease (prior myocardial infarction, stroke, or peripheral arterial disease) but no diabetes history. Patients were randomized to receive weekly subcutaneous semaglutide 2.4 mg or placebo, with a mean follow-up of 39.8 months. The trial demonstrated a 20% reduction in the primary composite endpoint of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke (hazard ratio 0.80, 95% CI 0.72-0.90).[1]
The trial also demonstrated improvements in multiple cardiovascular biomarkers independent of weight loss. Semaglutide reduced systolic blood pressure by 3.3 mm Hg, high-sensitivity C-reactive protein by 37.8%, and improved lipid profiles—all achieved on top of high baseline rates of evidence-based cardiovascular therapies (90% on statins, 86% on antiplatelet agents).[1] These pleiotropic effects likely contribute to cardiovascular risk reduction through mechanisms distinct from adiposity changes, supporting the reconceptualization of GLP-1 receptor agonists as cardiovascular disease-modifying agents rather than simply weight-loss medications.
Semaglutide's Cardiovascular Benefits: Independent of Weight Loss and BMI
One of the most surprising discoveries about semaglutide—a medication initially developed for diabetes and weight management—is that its cardiovascular benefits don't depend on how much weight patients lose. A landmark analysis of the SELECT trial revealed that patients who lost significant weight on semaglutide experienced similar cardiovascular protection to those who lost little or no weight. This finding fundamentally challenges our understanding of how this medication works and has important implications for who should receive it.
The SELECT trial enrolled 17,604 patients with a mean body mass index (BMI) of 33.3 and preexisting cardiovascular disease (prior heart attack, stroke, or peripheral artery disease) but no diabetes. Patients received either weekly subcutaneous semaglutide 2.4 mg or placebo for an average of 39.8 months. The trial demonstrated a 20% reduction in the primary composite endpoint of cardiovascular death, nonfatal heart attack, or nonfatal stroke (hazard ratio 0.80, 95% CI 0.72-0.90).
Weight Loss Doesn't Predict Cardiovascular Benefit
When researchers examined whether the amount of weight loss predicted cardiovascular outcomes, they found no linear relationship between weight loss at week 20 and subsequent risk of major adverse cardiovascular events (MACE) among semaglutide-treated patients. Cardiovascular outcomes were similar among patients who did or did not lose at least 5% of their baseline body weight. Even among the 5.5% of patients in the semaglutide group who actually gained weight, there appeared to be some cardiovascular benefit, though the effect was less pronounced—possibly due to medication non-adherence.
This stands in stark contrast to the placebo group, where weight loss was paradoxically associated with increased cardiovascular risk. The few patients receiving placebo who lost at least 5% of their baseline body weight had the highest rates of cardiovascular events. This suggests that substantial unintentional weight loss in placebo patients likely reflected underlying health problems—such as cancer, heart failure, or other serious conditions—rather than therapeutic benefit. This paradox highlights why comparing weight loss effects between treatment groups can be misleading.
Waist Circumference: A Modest Contributor
While overall body weight showed little relationship to cardiovascular outcomes, waist circumference reduction did show a modest association with benefit. Greater waist circumference reduction at week 20 was associated with lower subsequent MACE risk, suggesting that reduction in central adiposity (visceral fat around the organs) may contribute to cardiovascular protection. This makes biological sense, as visceral fat exerts greater adverse metabolic and inflammatory effects than peripheral fat stored elsewhere in the body.
However, even this relationship explained only 33% of semaglutide's treatment effect on MACE. In other words, two-thirds of the cardiovascular benefit occurred through mechanisms completely independent of fat loss. The stronger correlation between changes in waist circumference and body weight in the semaglutide group compared with placebo suggests that weight loss induced by semaglutide preferentially targets visceral adiposity—but this still accounts for only a minority of the cardiovascular protection.
Cardiovascular Benefits Across the BMI Spectrum
The cardiovascular benefits of semaglutide were independent of baseline adiposity measures, meaning patients across the full spectrum of BMI values experienced similar relative risk reductions. This is particularly important because it suggests that current prescribing restrictions based primarily on BMI thresholds may be too restrictive. Most patients treated for cardiovascular disease have a BMI of at least 27, suggesting that many patients could benefit from semaglutide regardless of their starting weight.
The early cardiovascular benefits observed in SELECT—with the treatment curves separating within the first few months—occurred well before substantial weight loss would be expected. This temporal relationship provides additional evidence that semaglutide's cardiovascular protection operates through rapid physiological changes beyond body weight reduction.
How Does Semaglutide Protect the Heart Without Weight Loss?
It's not entirely clear, however, several mechanisms likely explain semaglutide's cardiovascular benefits beyond adiposity reduction:
Direct effects on blood vessels and atherosclerosis: Semaglutide has been shown to improve endothelial function (the health of blood vessel linings) and affect atherosclerotic pathways directly, independent of weight changes.
Anti-inflammatory effects: Recent data suggest that GLP-1 receptor signaling in the brain may modulate systemic inflammation throughout the body. In the SELECT trial, semaglutide reduced high-sensitivity C-reactive protein (an inflammatory marker) by 37.8%—a reduction large enough to move patients from high cardiovascular risk (greater than 3 mg/dL) to intermediate risk (1-3 mg/dL).
Blood pressure and lipid improvements: Semaglutide reduced systolic blood pressure by 3.3 mm Hg and improved lipid profiles, all achieved on top of high baseline rates of evidence-based cardiovascular therapies (90% on statins, 86% on antiplatelet agents).
Independence from glucose control: In SELECT participants without diabetes, cardiovascular risk reduction was consistent across baseline HbA1c subgroups and across categories of HbA1c change from baseline to 20 weeks, confirming that the benefits don't depend on blood sugar improvements either.
Evidence from Patients with Diabetes
The independence of cardiovascular benefit from weight loss extends to patients with type 2 diabetes as well. A pooled analysis of the SUSTAIN 6 and PIONEER 6 trials, which studied semaglutide in diabetic patients, demonstrated that treatment effects on MACE remained similar when accounting for body weight changes. This finding was consistent across baseline BMI subgroups, indicating direct cardiorenal effects of semaglutide independent of weight loss.
Meta-analyses across multiple trials have confirmed these patterns. A 2025 systematic review of four randomized controlled trials involving 27,617 participants showed that semaglutide reduces MACE by 19% (relative risk 0.81, 95% CI 0.74-0.88), with additional benefits for cardiovascular death and nonfatal heart attack. Importantly, these benefits occurred despite varying degrees of weight loss across the different trials and patient populations.
Special Populations: Heart Failure
In patients with both obesity and heart failure with preserved ejection fraction (HFpEF), semaglutide's cardiovascular benefits appear particularly robust. A pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM trials examined 1,461 patients with HFpEF, most of whom had overweight or obesity. Treatment with semaglutide reduced the risk of the composite endpoint of cardiovascular death or worsening heart failure events compared to placebo.
Interestingly, the relative effects of semaglutide on this composite endpoint appeared more pronounced in participants with BMI of 35 or higher versus those with lower BMI, with a significant interaction between these subgroups. However, benefits were still observed across the BMI spectrum, and the effects were generally consistent across multiple subgroups, including those with mildly reduced versus preserved ejection fraction, and those receiving versus not receiving other heart failure medications like mineralocorticoid receptor antagonists and SGLT2 inhibitors at baseline.
Clinical Implications: Rethinking Who Should Receive Semaglutide
These findings have substantial implications for both clinical practice and healthcare policy. For clinicians and patients, it should not be assumed that lack of substantial weight loss on semaglutide would preclude the opportunity for improved cardiovascular outcomes. A patient who loses only 2-3% of their body weight may still experience the full cardiovascular benefit of the medication.
Furthermore, the demonstration of cardiovascular benefits across a broad range of adiposity levels, coupled with independence from weight loss magnitude, suggests that semaglutide and perhaps other GLP-1 receptor agonists should be reconceptualized as cardiovascular disease-modifying agents rather than solely medications for glycemic control or weight loss. Current prescribing restrictions, which are largely based on BMI thresholds or weight-loss targets, may not be appropriate, as patients with established cardiovascular disease might benefit regardless of their weight-loss response.
The FDA has recognized these cardiovascular benefits through multiple approvals. Wegovy (subcutaneous semaglutide 2.4 mg) is approved for reducing major adverse cardiovascular events in adults with established cardiovascular disease and obesity or overweight—the first weight-management medication to receive this indication. Ozempic (subcutaneous semaglutide) is approved for reducing MACE in adults with type 2 diabetes and established cardiovascular disease, as well as reducing the risk of sustained kidney function decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease. Rybelsus (oral semaglutide) is approved for reducing MACE in adults with type 2 diabetes.
Balancing Benefits and Tolerability Of GLP1s
While semaglutide demonstrates clear cardiovascular benefits independent of weight loss, treatment discontinuation rates merit consideration. Meta-analyses show that semaglutide increases the likelihood of discontinuing treatment (relative risk 1.67, 95% CI 1.27-2.21), primarily due to gastrointestinal adverse events like nausea, vomiting, and diarrhea. However, this must be weighed against the medication's ability to reduce serious adverse events overall (relative risk 0.91, 95% CI 0.88-0.94). The trade-off between tolerability and cardiovascular protection represents an important consideration for patient counseling and shared decision-making.
The Future Of Ozempic and GLP1s
Future research should focus on the broader potential mechanisms of cardiovascular protection with GLP-1 receptor agonists. Studies incorporating formal measures of body composition—distinguishing between fat mass and muscle mass—could provide additional insights, as weight loss may result from reductions in both, and the degree of each may differ between individuals and between drivers of weight loss.
The longest and largest trial of GLP-1 receptor agonists in patients with atherosclerotic cardiovascular disease has demonstrated that semaglutide results in improved cardiovascular outcomes independent of baseline adiposity and over a wide range of treatment-induced weight loss. This evidence supports expanding access to semaglutide for cardiovascular protection beyond traditional weight-loss indications, potentially benefiting millions of patients with established cardiovascular disease regardless of their ability to lose weight on the medication.
References:
Semaglutide Cardiovascular Outcomes Trials
- Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes (SELECT). New England Journal of Medicine. 2023.
https://www.nejm.org/doi/full/10.1056/NEJMoa2307563 - Lingvay I, Deanfield J, Kahn SE, et al. Semaglutide and Cardiovascular Outcomes by Baseline HbA1c and Change in HbA1c in People With Overweight or Obesity but Without Diabetes in SELECT. Diabetes Care. 2024;47(8):1360-1369.
https://diabetesjournals.org/care/article/47/8/1360/156810 - Deanfield J, Verma S, Scirica BM, et al. Semaglutide and Cardiovascular Outcomes in Patients with Obesity and Prevalent Heart Failure: A Prespecified Analysis of the SELECT Trial. The Lancet. 2024;404(10454):773-786.
https://doi.org/10.1016/S0140-6736(24)01498-3 - Marso SP, Daniels GH, Brown-Frandsen K, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes (SUSTAIN-6). New England Journal of Medicine. 2016.
https://www.nejm.org/doi/full/10.1056/NEJMoa1607141 - McGuire DK, Marx N, Mulvagh SL, et al.; SOUL Study Group. Oral Semaglutide and Cardiovascular Outcomes in High-Risk Type 2 Diabetes. New England Journal of Medicine. 2025.
https://www.nejm.org/doi/10.1056/NEJMoa2501006 - American College of Cardiology. SOUL Trial Summary: Oral Semaglutide Cardiovascular Outcomes. 2025.
https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2025/03/27/15/04/soul - American Diabetes Association. Press Release: Oral Semaglutide Significantly Improves Cardiovascular Outcomes (SOUL). 2025.
https://diabetes.org/newsroom/press-releases/oral-semaglutide-significantly-improves-cardiovascular-outcomes-individuals
Review Articles & Meta-Analyses
- Nauck MA, Quast DR. Cardiovascular Safety and Benefits of Semaglutide in Patients With Type 2 Diabetes: Findings From SUSTAIN 6 and PIONEER 6. Frontiers in Endocrinology. 2021;12:645566.
https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2021.645566 - Sadraei S, Aarabi A, Rajai Firouzabadi S, et al. Cardiovascular Benefits of Semaglutide: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Cardiovascular Disorders. 2025;25(1):10.1186/s12872-025-05278-3.
- Cleto AS, Schirlo JM, Beltrame M, et al. Semaglutide Effects on Safety and Cardiovascular Outcomes in Patients With Overweight or Obesity: A Systematic Review and Meta-Analysis. International Journal of Obesity (Lond). 2025;49(1):21-30.
Other Relevant Clinical & Review Articles
- Knop FK, Aroda VR, do Vale RD, et al. Oral Semaglutide 50 mg Taken Once Per Day in Adults With Overweight or Obesity (OASIS-1): A Randomised, Double-Blind, Placebo-Controlled Phase-3 Trial. Lancet. 2023;402(10403):705-719.
- Elmaleh-Sachs A, Schwartz JL, Bramante CT, et al. Obesity Management in Adults: A Review. JAMA. 2023;330(20):2000-2015.
- Kosiborod MN, Deanfield J, Pratley R, et al. Semaglutide Versus Placebo in Patients With Heart Failure and Mildly Reduced or Preserved Ejection Fraction: Pooled Analysis of SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM Randomised Trials. Lancet. 2024;404(10456):949-961.
FDA References
- U.S. Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations — Ozempic (Semaglutide). (FDA Orange Book search entry)
- U.S. Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations — Rybelsus (Semaglutide).
- U.S. Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations — Wegovy (Semaglutide).
(These are official FDA Orange Book entries and can be accessed by searching the drug names on https://www.fda.gov/drugs/orange-book)
Still Have Questions? Stop Googling and Ask Dr. Alo.
You’ve read the science, but applying it to your own life can be confusing. I created the Dr. Alo VIP Private Community to be a sanctuary away from social media noise.
Inside, you get:
-
Direct Access: I answer member questions personally 24/7/365.
-
Weekly Live Streams: Deep dives into your specific health challenges.
-
Vetted Science: No fads, just evidence-based cardiology and weight loss.
Don't leave your heart health to chance. Get the guidance you deserve. All this for less than 0.01% the cost of health insurance! You can cancel at anytime!
[👉 Join the Dr. Alo VIP Community Today]